Custom recombinant proteins
Design, production and purification of recombinant proteinsAntigen relevance is the most important parameter when developing a therapeutic or a diagnostic tool.
CER Groupe offers a comprehensive range of solutions to optimize the design, production and purification of recombinant proteins, in order to maximize their reliability in your application.

Our team of experts has an extensive track record of successful projects on two expression (USP) platforms:
- Prokaryotic: E. coli
- Eukaryotic: high-density, suspension-growing, overexpressing mammalian cells (HEK293-6E cells licensed from NRC).
Our integrated solutions for recombinant protein production are characterized by a high success rate, even for challenging proteins. The bioproduction unit, which is based in Belgium, is trained to propose comprehensive strategies and deliver high-quality results in total confidentiality and in accordance with proposed timelines, from development to routine supply.
Manufacturing capacity
Our manufacturing capacity covers the range required for most research, diagnostic and pre-clinical developments, from mg to g quantities (10 L scale stirred-tank bioreactor). Following expression, the biological products are purified, conditioned and stabilized using tailored strategies.
Finally, robust QC methods are used to characterize the final product, from a standard evaluation of quantity and purity to advanced characterization for activity and structural characteristics and to guarantee that the product meets your specifications.
Our integrated services include:
- bioinformatic analysis
- antigen design
- gene optimization and cloning
- protein/cell engineering
- transient expression in E. coli and mammalian cells
- stable cell line development
- small scale feasibility studies
- isotope labeling
- artificial proteins (polyepitope / concatemer)
- optimization of solubilization and refolding of inclusion bodies
- USP/DSP optimization
- freeze-drying platform for development and routine processing
- analytical services and bio-assays
Key figures
>85% success rate
>200 proteins produced
Up to 180 mg/L using HEK293-6E cell line
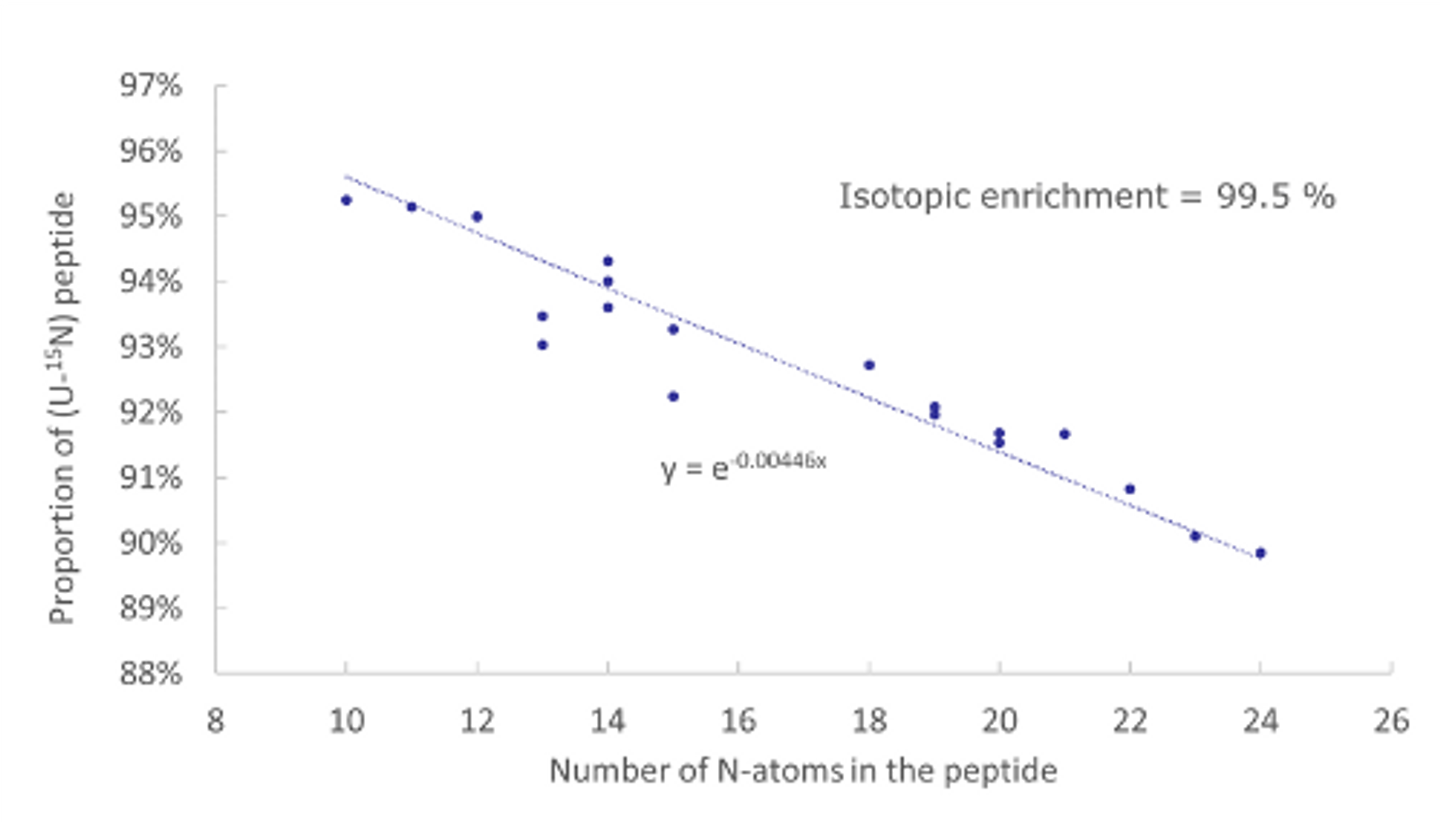
CASE STUDY
Custom stable isotope-labelled concatemer
Design, production and characterization of stable isotope-labelled concatemer as internal standard for multiple proteins quantification by MS.
