Custom viral vectors
Promising tools for cell and gene therapy applicationsViral vectors are recombinant viruses used to deliver genetic material into target cells and are used for research, biomanufacturing and therapeutic applications.
They represent promising tools for cell and gene therapy applications, but they are very complex by nature in terms of development and manufacturing.
There is currently a gap between gene therapy developers, who are primarily focused on the targeted indication, and CDMO, who are mostly focused on GMP production. As a result, non-clinical development lead times and risks are increased. Additionally, potential lack of efficiency and low productivity may lead to impaired production capacities and increased costs, in a market that needs to improve its accessibility.
Integrated services
In partnership with the viral vector platform from the GIGA Institute at Liege University, CER Groupe offers integrated services for lentivirus and adeno-associated virus (AAV), the two most used types of viral vectors in clinical trials.
The service covers all the critical steps of non-clinical development, in order to mitigate risks for gene therapy developers and accelerate development. This is done by using a qualified environment, processes, and characterization methods, by taking potential IP constraints into account, and by facilitating subsequent tech transfer to GMP CDMO.
By combining partners’ expertise in design and rigorous screening, biomanufacturing/characterization capabilities and non-clinical development services, our solution fills the existing gap and ensures an efficient and transparent clinical project transfer.
Key figures
74% of gene therapy clinical trials use AAV or lentivirus in H1 2022
>10^15 gc batch production capacity
>300 viral vectors developed per year by GIGA

The typical project steps are :
- design of viral vectors
- small-scale production and selection of viral vectors
- production and purification of viral vectors
- scaling up of production for the pre-clinical stage
- non-clinical in-vitro and in-vivo characterization
We also have the expertise to custom produce other types of recombinant or wild-type viruses in our facilities, up to BSL-3 level, on various animal and human cell types, for research, diagnostic or non-clinical applications. As an example, our expertise in virus amplification is also used to generate in-vivo models that are representative of human viral infection (IAV, co-infection IAV, SARS-CoV-2, RSV, etc.) for conducting pre-clinical studies in our facilities.
Are you developping a gene-therapy product?
Rely on our viral vector platform, which provides a unique and fully integrated solution for efficient lentivirus and AAV design and development and mitigating project risks.
CER Groupe's viral vector platform provides optimal service for pre-clinical scale production of gene therapy candidates, as well as qualified product characterization methods and non-clinical development solutions.
Our practices are easily scalable thanks to our processes, which align with industrial-scale production requirements. This makes it possible to optimize your investment by facilitating tech transfer to GMP CDMO and accelerating global development timelines.
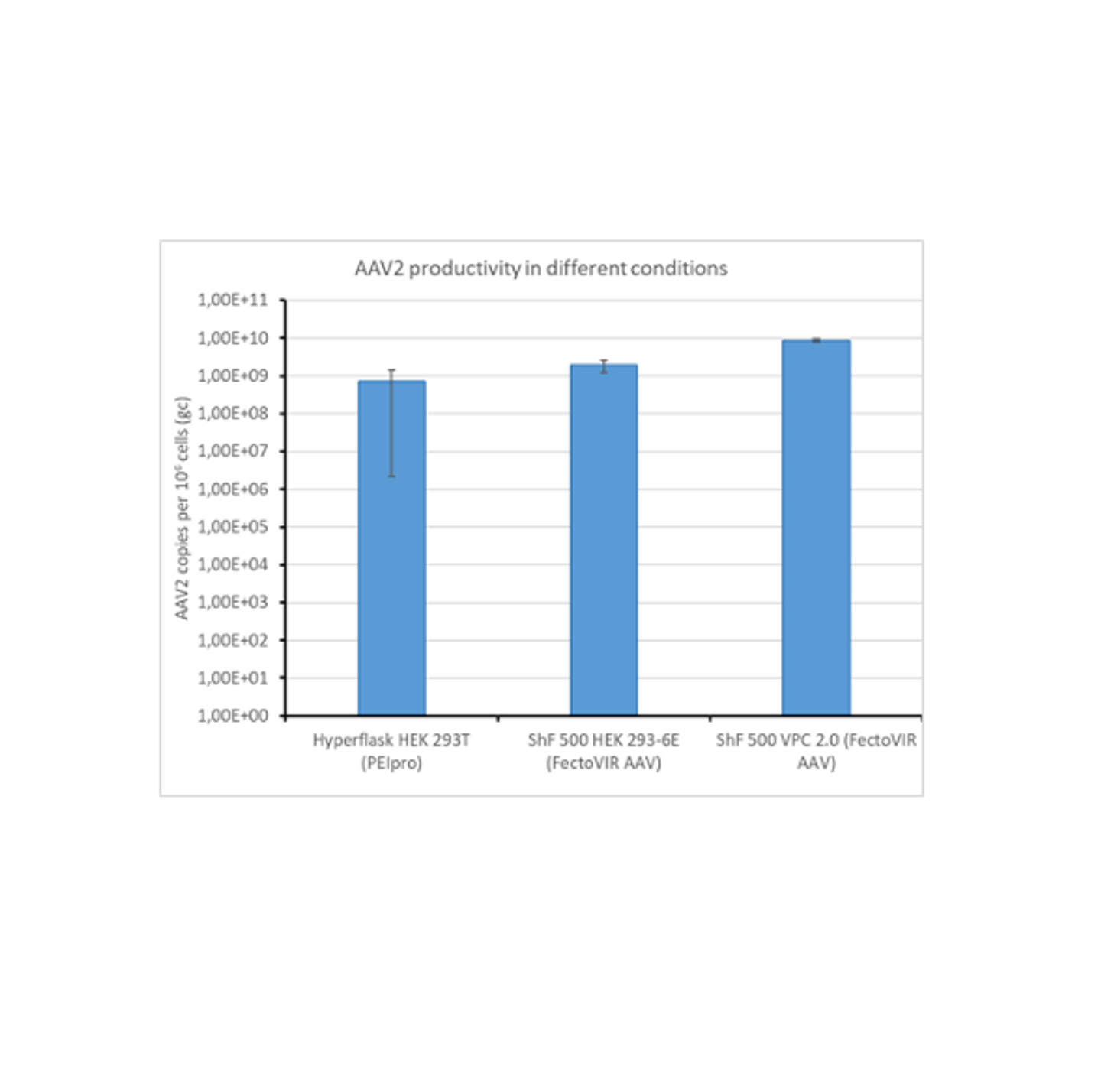
CASE STUDY
Pre-clinical-scale production, purification and characterization of AAV2 viral vectors
Viral vectors are powerful biological products with great potential for gene therapy. However, due to their complexity and production process, their current productivity is low.
