
A partner for your developments in bioproduction, from R&D to pre-clinical validation.
CER Groupe, a research center recognized for its CRO services in pre-clinical and bioproduction, deploys its integrated bioproduction offer to support biotechnology and diagnostic companies from concept development to pre-clinical validation and routine production.
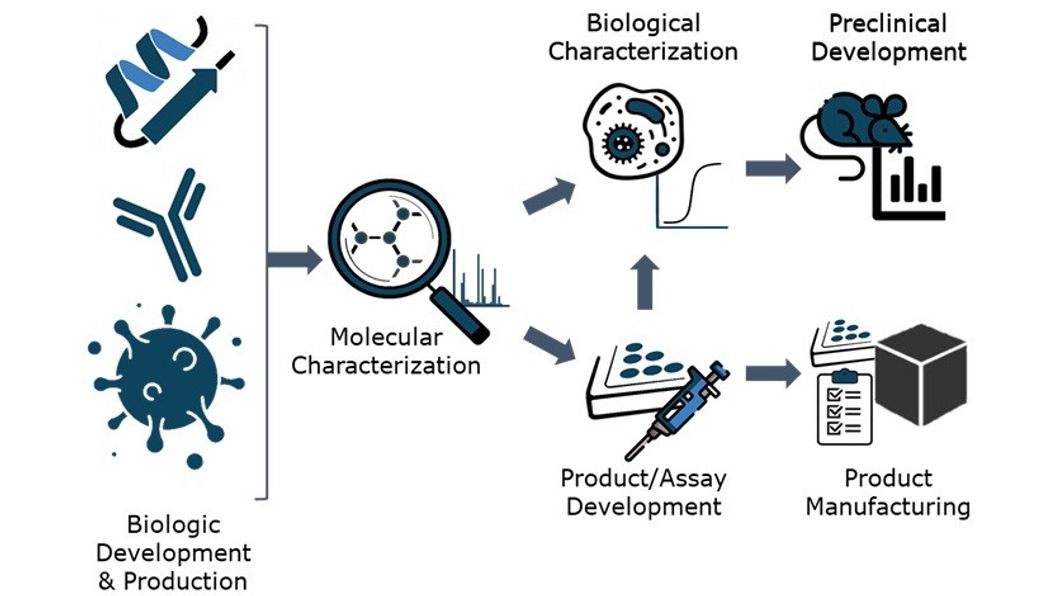
Our bioproduction capabilities
CER Groupe develops and produces poly- and monoclonal antibodies, recombinant proteins, and AAV and lentivirus-type viral vectors. The group specializes in recombinant production from mammalian cells, in particular using the HEK293-6E cell line, which demonstrates exceptional performance in the production of proteins through transient transfection. The offer covers both high-throughput development/screening scale and the production of selected candidates in a stirred bioreactor and covers needs under finely regulated conditions, thus ensuring optimal and reproducible product quality for pre-clinical or diagnostic applications.
The analytical capabilities of CER Groupe make it possible to characterize the intrinsic properties of your biological products using validated and widely proven methods, and thus verify compliance with your specifications, both at the molecular and biological level. Also, thanks to our expertise in analytical development and our state-of-the-art platforms, new custom characterization methods can be developed quickly, for example, to assess the in-vitro activity of a therapeutic candidate. The information obtained during these steps also makes it possible to guide product/process optimization, to quantify productivity gains, and to verify the quality of the product after optimizing the expression or purification of the candidate in question.
What does integrated bioproduction mean?
On one hand, in the context of therapeutic candidates, after the preliminary evaluation of biological activity, CER Groupe can ensure pre-clinical development, proof of efficacy and the study of the mechanism of action in full, using models adapted to pharmacology and toxicity studies under GLP conditions. On the other hand, diagnostic candidates can serve as a basis for developing high-quality reagents, such as next-generation conjugate antibodies, and then developing tests that can be produced under ISO 9001 certification.
By way of example, the diagram below represents certain activities carried out around the theme of SARS-CoV-2. In short, CER Groupe was able to demonstrate its ability to produce and characterize very high-quality antigens, to use them to develop highly refined antibodies, to demonstrate their neutralizing properties in vitro, to develop and validate analytical methods, and finally, to exploit these tools to characterize the properties of various candidate vaccines in a very short period of time.
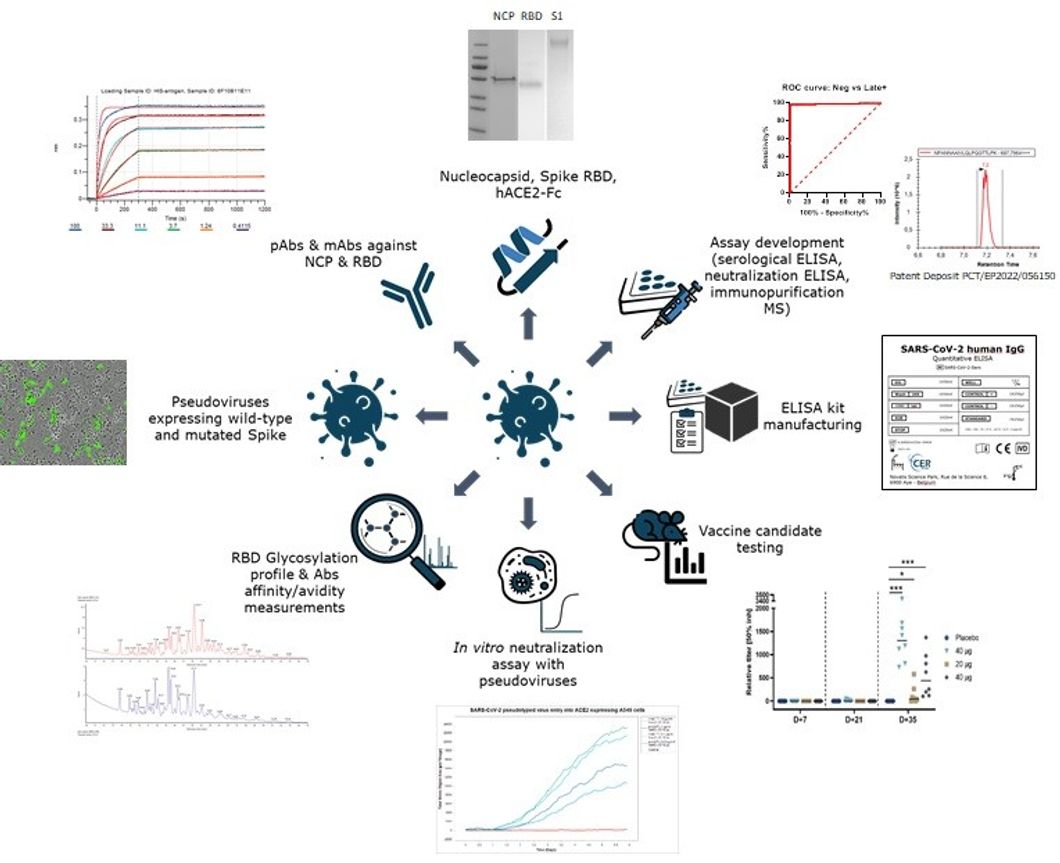
The evolution of our development, production, and characterization capabilities, all the way up to their integration into pre-clinical activities, now enables CER Groupe to offer complete support for a wide variety of molecules and during development projects for therapeutic candidates representing real technical challenges, by offering a personalized, flexible, and agile approach. These capabilities are made possible thanks to the 15,000 m² of operational infrastructure available to CER Groupe and, above all, to the nearly 160 employees at the research center, including 50 MSc/VMD/PhD. As teams are fully integrated, project monitoring is simplified and development times are minimized. Finally, these services are carried out according to quality standards that are adapted to the needs of our partners, from ISO 9001 to GMP-like.
Let's keep in touch
Activity is booming at CER Groupe. Follow the company on LinkedIn to stay up to date with the latest announcements. Do you have a project that could benefit from our integrated bioproduction offer? Contact us to learn how CER can support you and mitigate the risk of your developments!
Other news



